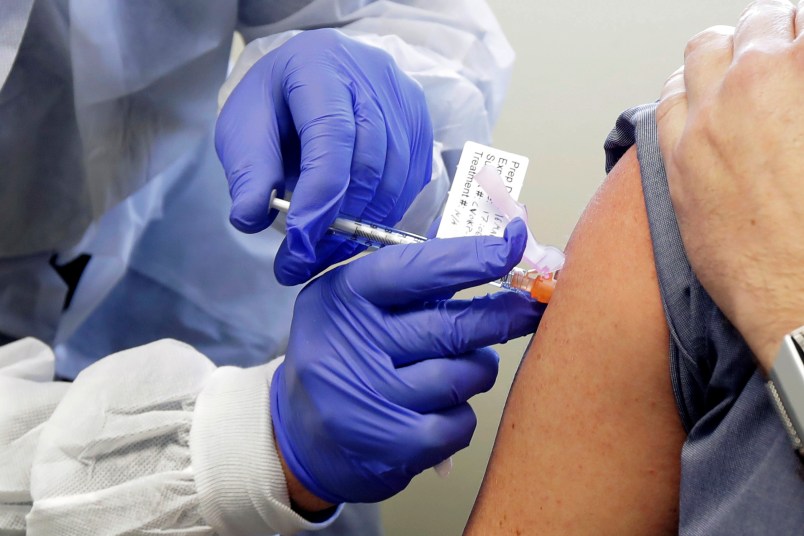
A group of drug companies racing against each other to develop coronavirus vaccines, are planning to release a statement early next week pledging that they will not prematurely release a vaccine amid political pressure to deliver on a presidential election timeline that is now just two months away.
A copy of the companies’ draft statement obtained by the Wall Street Journal says the drugmakers will submit applications for government emergency-use authorization or vaccine licensing based on “substantial evidence of safety and efficacy” from advanced clinical trials conducted under Food and Drug Administration guidance.
The announcement of the pledge comes as concerns mount that a COVID-19 vaccine is being jammed through federal regulation to deliver a Trump campaign victory ahead of the election.
The companies have said they will look at Phase 3 trials, which are designed to demonstrate the efficacy of the shots at reducing rates of symptomatic COVID-19 compared with unvaccinated people.
The manufacturers who have reportedly signed the letter include Pfizer, Moderna, Johnson & Johnson, GlaxoSmithKline and Sanofi.
The news comes as doubts swell regarding the safety and efficacy of a vaccine that has faced a sped up timeline. Vaccine development usually takes years, yet Covid-19 shots are progressing at a rapid pace as companies and countries rush to stop the spread of the coronavirus.
Several of the companies have already embarked on large clinical trials that include at least 30,000 people. There was some discussion in recent weeks about possible emergency-use authorization or approval.
Last week, the CDC told state officials that they should prepare to launch COVID-19 vaccination campaigns by November.
The timeline coincides within days of the presidential elections on Nov. 3. Last month, President Donald Trump tweeted a conspiracy meant to pressure and simultaneously undermine the FDA by suggesting that a “deep state” within the agency was intentionally delaying trials for a vaccine to deny him a victory that would likely boost his chances ahead of the election.
Those remarks have intensified concern by lawmakers, with some states even refusing to distribute a vaccine if it is not properly vetted or that appears to cling too closely to Trump’s political schedule.
While the federal government has pledged to deliver 300 million doses by January 2021, the FDA has said it won’t approve a shot for coronavirus unless there is evidence showing it is 50% effective compared with a placebo.
But the credibility of the FDA is in repair, after it recently hastily authorized convalescent plasma to treat Covid-19 patients.
Earlier this summer, the FDA was also forced to revoke the emergency-use authorization of hydroxychloroquine, an antimalarial touted by Trump following concerns about its safety and efficacy as treatment.
FDA commissioner Stephen Hahn was on the receiving end of Trump’s earlier attack on the vaccine’s slower pace when he offered the glowing review of the treatment — he later apologized for presenting an overzealous picture that raved of the treatment’s effectiveness.